Bond Angle of Cyclohexane
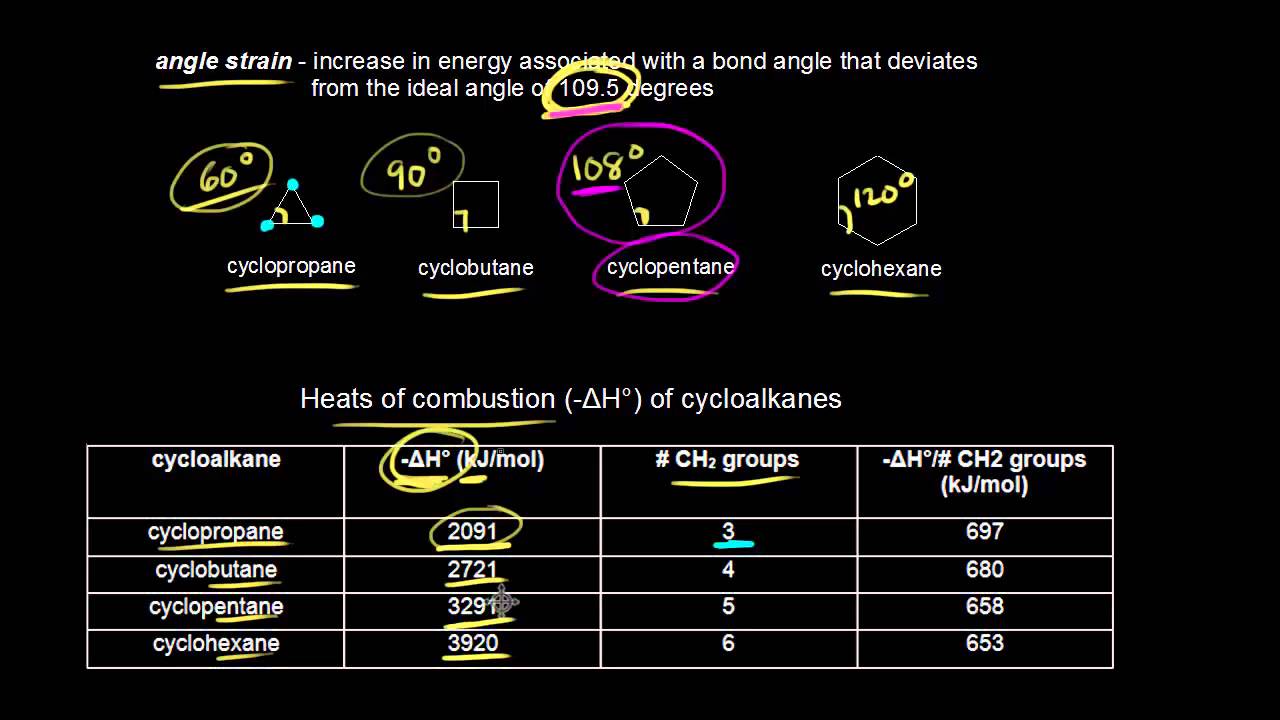
Since cyclopropane has a carbon-carbon bond angle of 60 o it has the highest ring strain among all cycloalkanes. Molecular geometry and mathematical interpretation The geometry of a set of atoms or molecules can be described by Cartesian coordinates of the atoms or internal coordinates formed from a set of bond lengths bond angles and dihedral angles.

Convert Newman Projection Of Cyclohexane To Bond Line Chemistry Textbook Chemistry Lessons Study Chemistry
The H-C-H bond angle is 115 whereas 106 is expected as in the CH 2 groups of propane.

. A few other uses of saturated hydrocarbons are listed below. Structural formula shows the minimal detail that shows the arrangement of atoms in a. Oriented close to the axis running through the centre of a cyclohexane ring as opposed to equatorial.
Indicate whether each of the following molecules is polar or nonpolar. Based on previous synthetic methods in our lab as shown in Scheme 2 6519 g propionic acid 25 g cyclohexane 01 ml 2-methylpyridine and 08 g heteropolyacid IL catalyst were mixed in a 500 ml three-necked flaskThen 25 g H 2 O 2 50 was added slowly through a 100 ml constant pressure funnel at 65 C 54 kpa with a magnetic stirring for 4 h. A Newman projection useful in alkane stereochemistry visualizes the conformation of a chemical bond from front to back with the front atom represented by a dot and the back atom as a circle.
Cyclohexane C 6 H 12 Although the chair conformation is able to achieve ideal angles the unstable half-chair conformation has angle strain in the. The molecular geometry is given in parentheses. Circle the following molecules that have the R configuration A.
Bond flanges should be designed to be as narrow as possible without sacrificing the structural integrity of the assembly. The front atom is called proximal while the back atom is called distalThis type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms. Small rings such as three and four membered rings have significant angle strain resulting from the distortion of the sp 3 carbon bond angles from the ideal 1095º to 60º and 90º respectively.
In small-ring cyclic compounds ring strain can be a major contributor to thermodynamic instability and chemical reactivity. Cyclohexane is clearly the most stable lower potential energy of the four isomers depicted. Typically bond flange widths should range in size from 16 to 25 mmA minimum flange width of 6 mm may be used on smaller parts such as spoilersA minimum of 3 mm clearance from the tangent should be allowed between the edge of the inner panel and the.
This angle strain often enhances the chemical reactivity of such compounds leading to ring cleavage products. Cyclohexane C 6 H 12 Cyclopropane C 3 H 6. In the bond line angle formula each covalent bond between carbon-carbon or carbon-heteroatom is Q.
PH2Cl trigonal pyramidal with P at the apex bSO3 trigonal planar with S in the center position cCH2Cl2 tetrahedral with C in the center position dCCl4 tetrahedral with C in the center position. What are the Uses of Saturated Hydrocarbons. Alkanes are widely used as fuels heating oils and solvents.
We present a detailed and comprehensive picture of the photophysics of thermally activated delayed fluorescence TADF. Among these angle strain and eclipsing strain bond orientation are severe in small rings such as cyclopropane and. The resulting catalysts were collected by centrifugation and washed with a cyclohexaneethanol mixture.
R indicates that a clockwise circular arrow that goes from higher priority to lower priority crosses. Given a set of atoms and a vector r describing the atoms positions one can introduce the concept of the energy as a. The approach relies on a few-state model parametrized ab initio on a prototypical TADF dye that explicitly accounts for the nonadiabatic coupling between electrons and vibrational and conformational motion crucial to properly address.
After drying the catalysts were annealed at 130 C for 6 h in Ar 100 sccm in a home.

Cyclohexane Chair Flip Summary Of How To Draw A Ring Flip Chemistry Student Learning Organic Chemistry

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Chemistry Chemistry Labs

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Teaching Chemistry Chemistry Lessons
Stability Of Cycloalkanes Organic Chemistry Books Organic Chemistry Chemistry
Comments
Post a Comment